Structural Magnetic Resonance Imaging (sMRI)
A Brief History
Magnetic resonance imaging (MRI) is a non-invasive diagnostic tool used to produce detailed images of the body's internal structures. The origins of MRI can be traced back to the early 20th century when physicists discovered nuclear magnetic resonance (NMR), a phenomenon in which atomic nuclei emit radio signals when placed in a magnetic field. In the 1970s, Raymond Damadian, a physician and medical researcher, discovered that different tissues in the body have unique NMR signals, leading to the development of the first MRI machine. In 1983, the first clinical MRI scanner was installed at the University of Aberdeen in Scotland, and since then, MRI has become an essential tool in modern medicine. The development of newer MRI techniques such as functional MRI (fMRI), diffusion tensor imaging (DTI), and magnetic resonance spectroscopy (MRS) have expanded the capabilities of MRI, making it a valuable tool for diagnosis, research, and treatment planning. Today, MRI is widely used in hospitals and clinics worldwide, and ongoing research continues to improve its capabilities and accuracy.
Physics of MRI

The physics underlying MRI is based on the behavior of atomic nuclei, specifically the protons (hydrogen ions) found in water molecules in the body. All soft tissue contains water including hydrogen. When a person is placed inside the MRI machine, the magnetic field causes the protons to align themselves along the direction of the field.
Applying a brief radio frequency (RF) pulse to a brain, horizontal to the magnetic filed that aligned the protons, forms a second magnetic field that pushes the aligned protons over onto their sides, generating a movement called “precession” (like rotation of the earth). When the second horizontal magnetic field is turned off, the synchronously spinning protons “relax” (standing up again). The relxation of the protons release the absorbed energy as a radio signal, which is detected by the MRI machine and used to construct an image.
Different tissues in the body have unique magnetic properties, and therefore, their protons absorb and release energy at different rates. This results in differences in the strength and timing of the radio signals emitted by different tissues, allowing for the creation of detailed images that distinguish between various types of tissues.
Types of sMRI
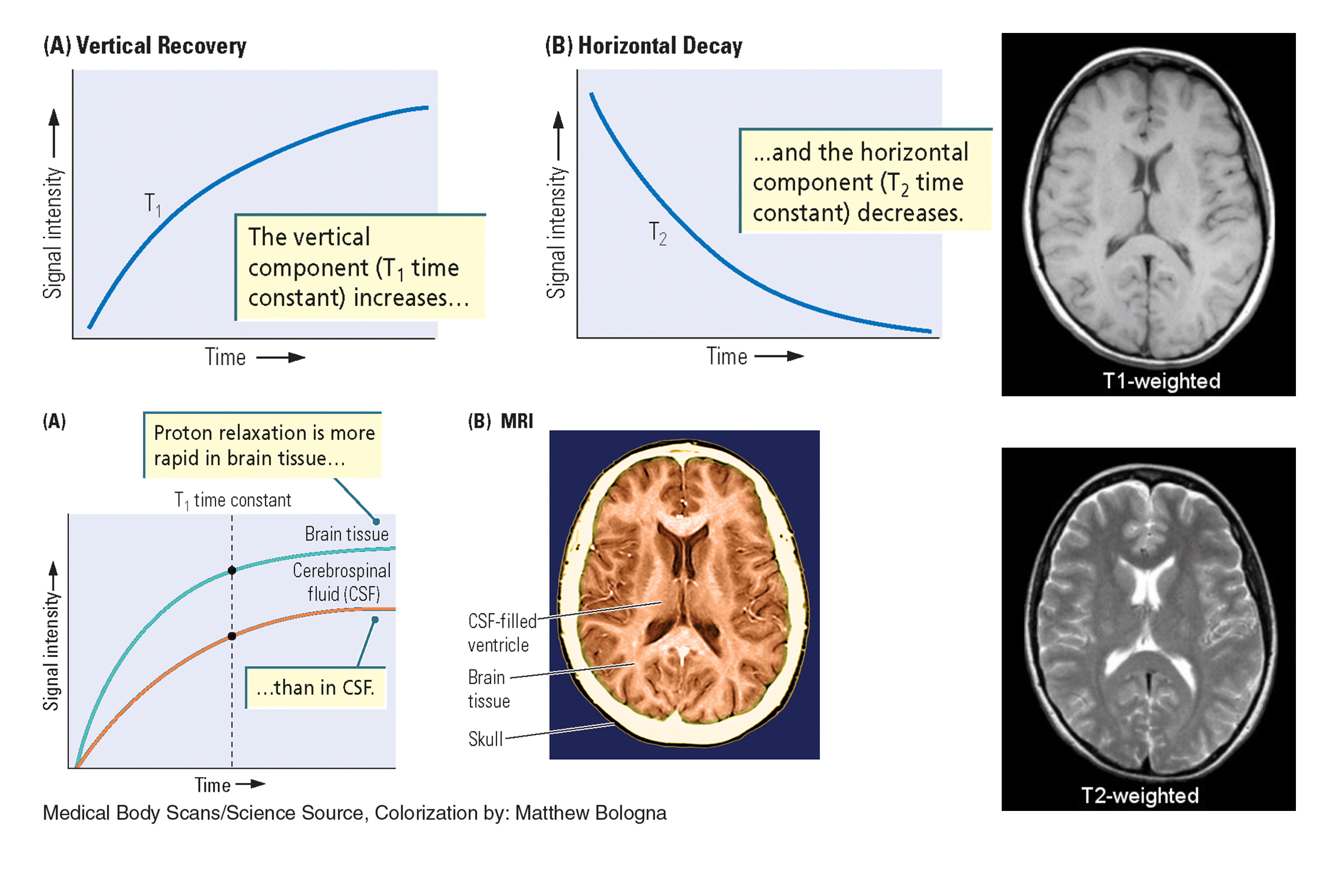
T1w & T2w MRIs
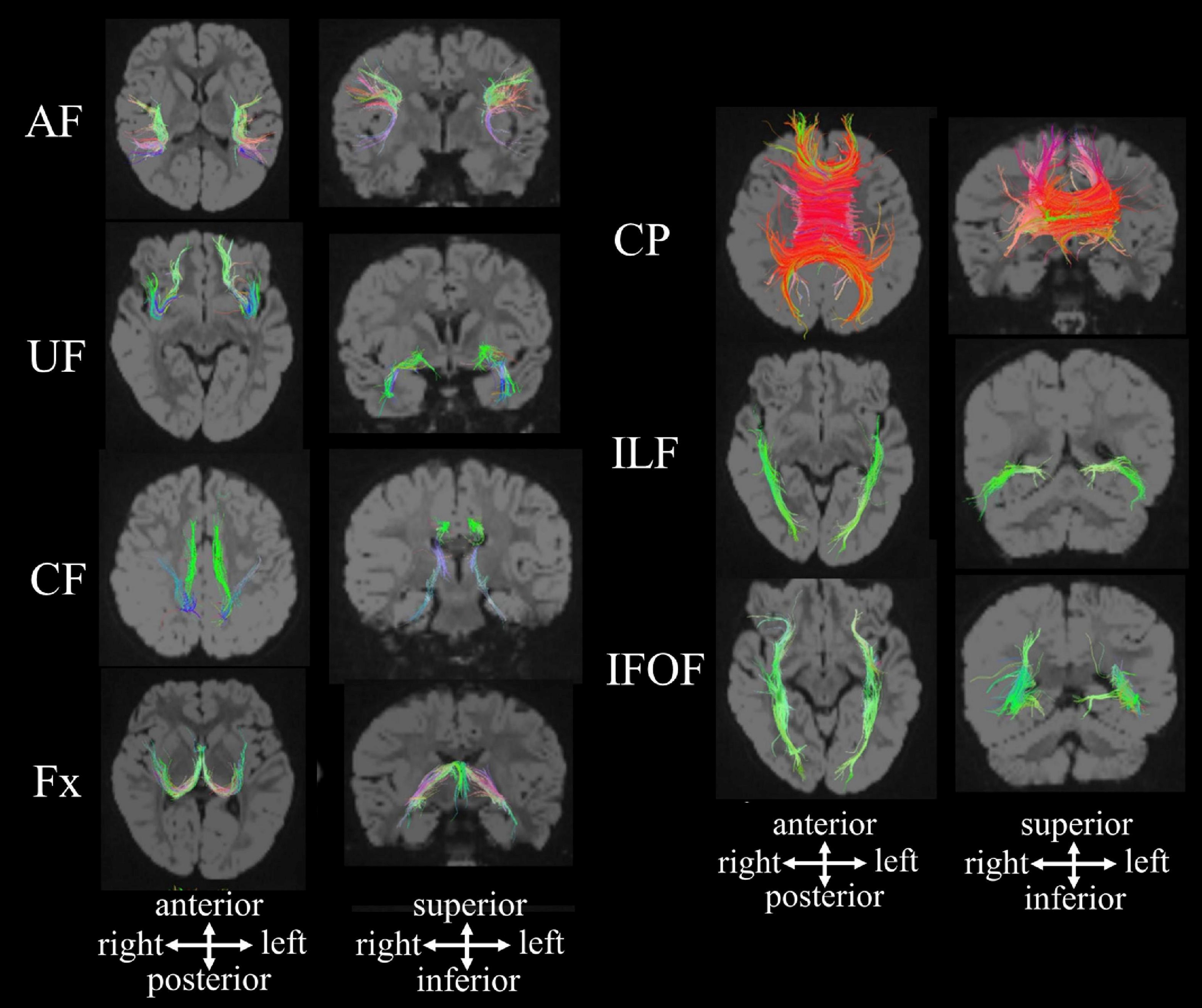
Diffusion Tensor Imaging (DTI)
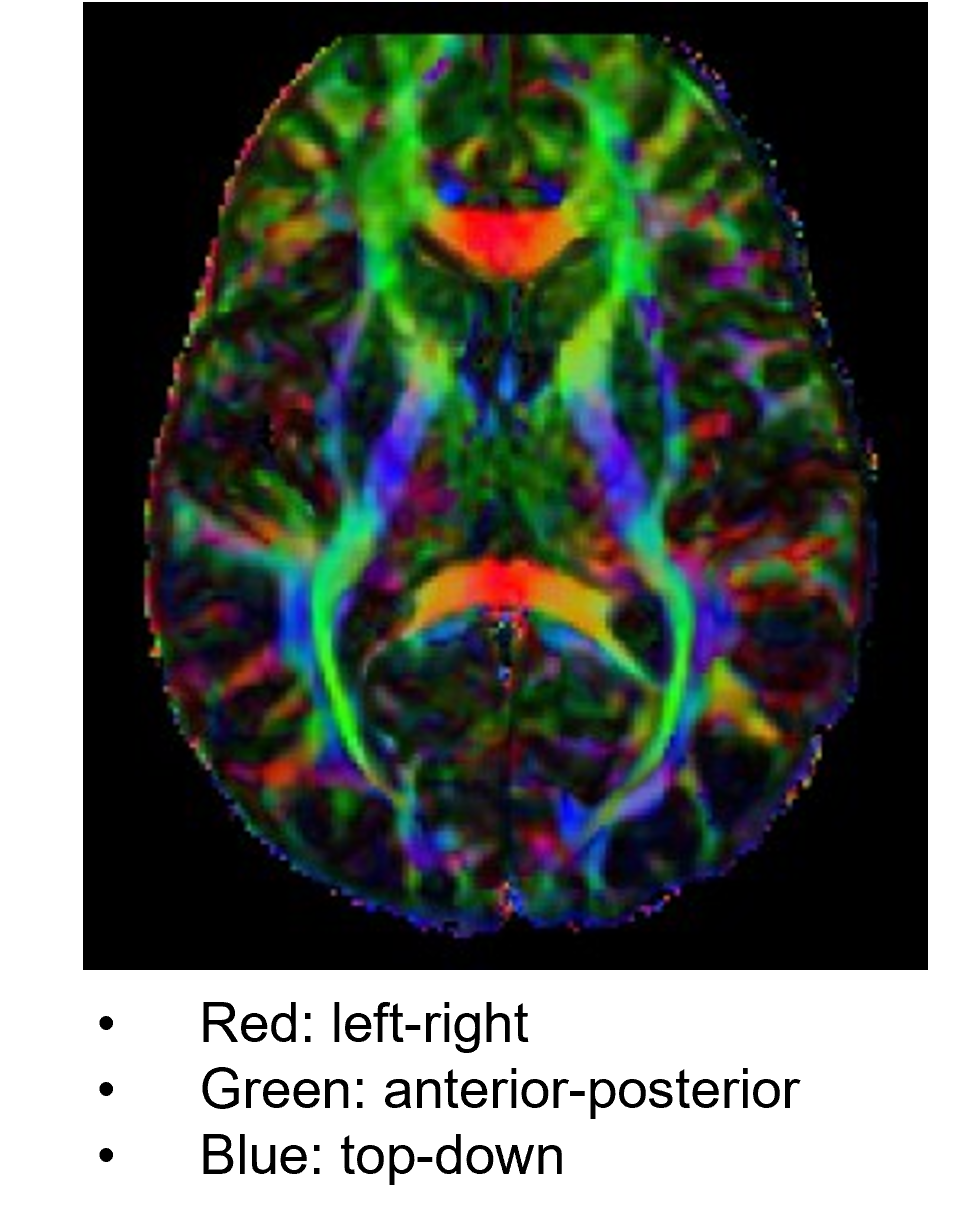
DTI is an MRI method that images fiber pathways by detecting directional movements of water molecules in ventricles. Movement of water molecules in nerve fibers tends to follow the longitudinal axis, a property called fractional anisotropy (FA). With DTI, we can detect degeneration of axons and distortion of and damage to fibers.
The information of white matter fiber tracts obtained from DTI can be also analyzed in combination with T1w/T2w MRIs, fMRI, and EEG/MEG measures. DTI images are often displayed in R-G-B colors representing strengths of different directional tensors. DTI is one of the central MRI measures of Human Connectome Project (HCP).
Magnetic Resonance Spectroscopy (MRS)
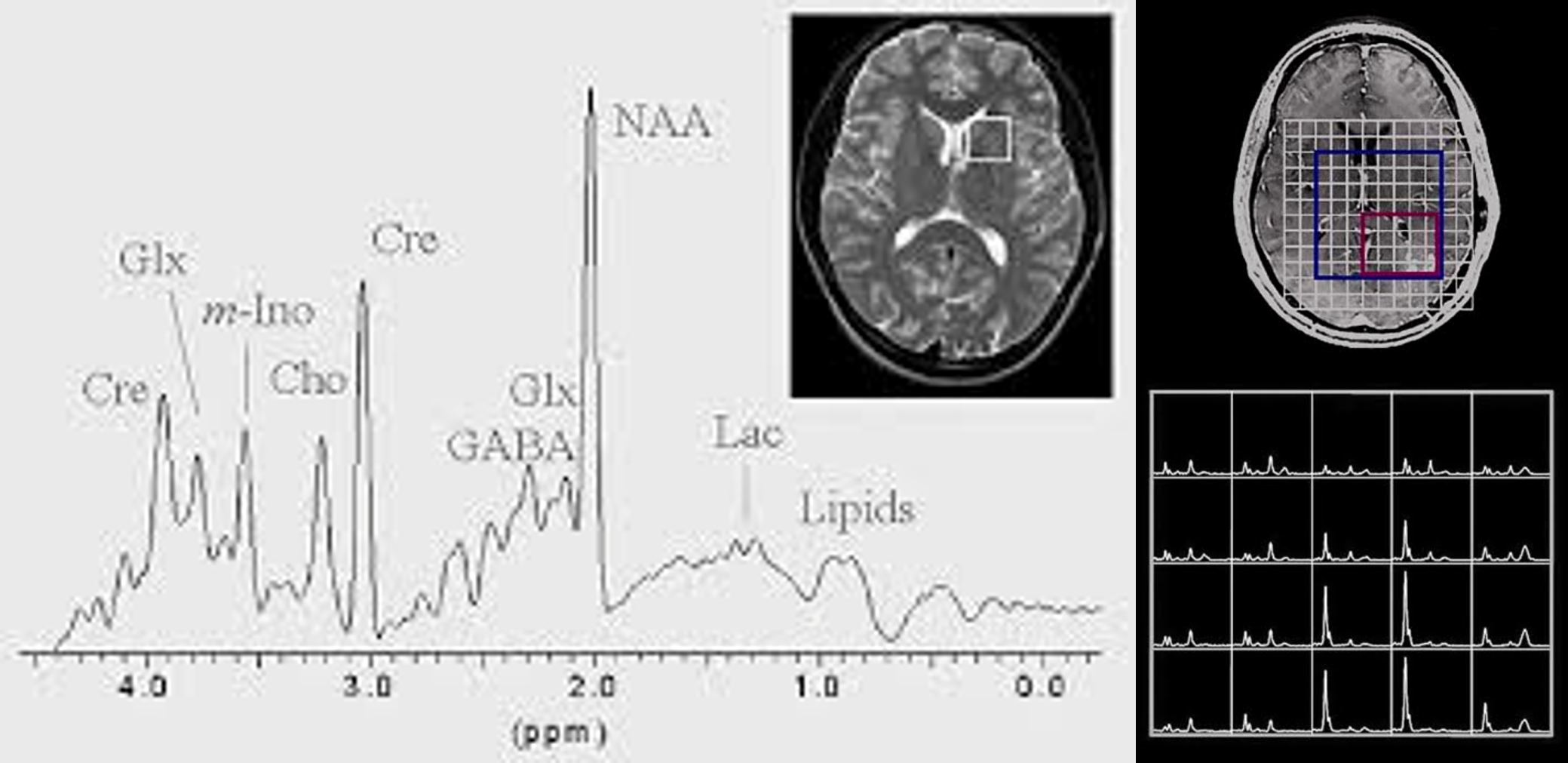
MRS uses a powerful magnetic field and radio waves to produce detailed information about the chemical composition of tissues in the body. Specifically, it measures the relative concentrations of various metabolites, such as amino acids, neurotransmitters, and lipids, by detecting the unique magnetic resonance signals produced by each of these molecules. MRS is often used in medical research and clinical practice to investigate a range of conditions, including cancer, neurodegenerative disorders, and psychiatric illnesses.
Analysis of sMRI
Although MEG and EEG represent different physical measures of neural activities (i.e., magnetic fields vs. electrical voltage), their signals share largely common features. They are direct and non-invasive measure of neural activities recorded at a high sampling rate (~1000 Hz). Therefore, all the EEG analysis techniques can be applied to MEG data in the time, frequency, time-frequency, and spatial domains.
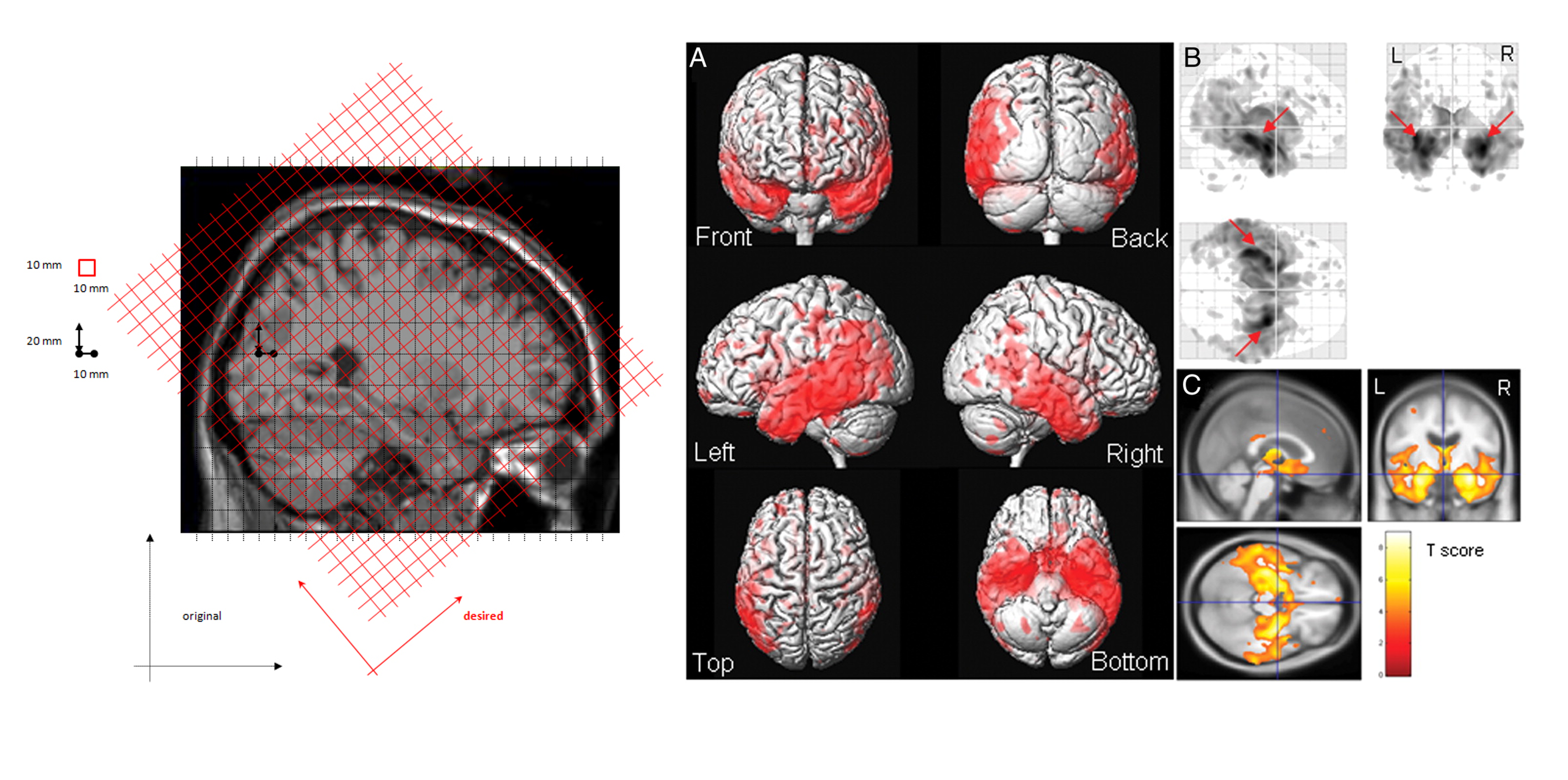
Volume (Voxel Based Morphometry [VBM])
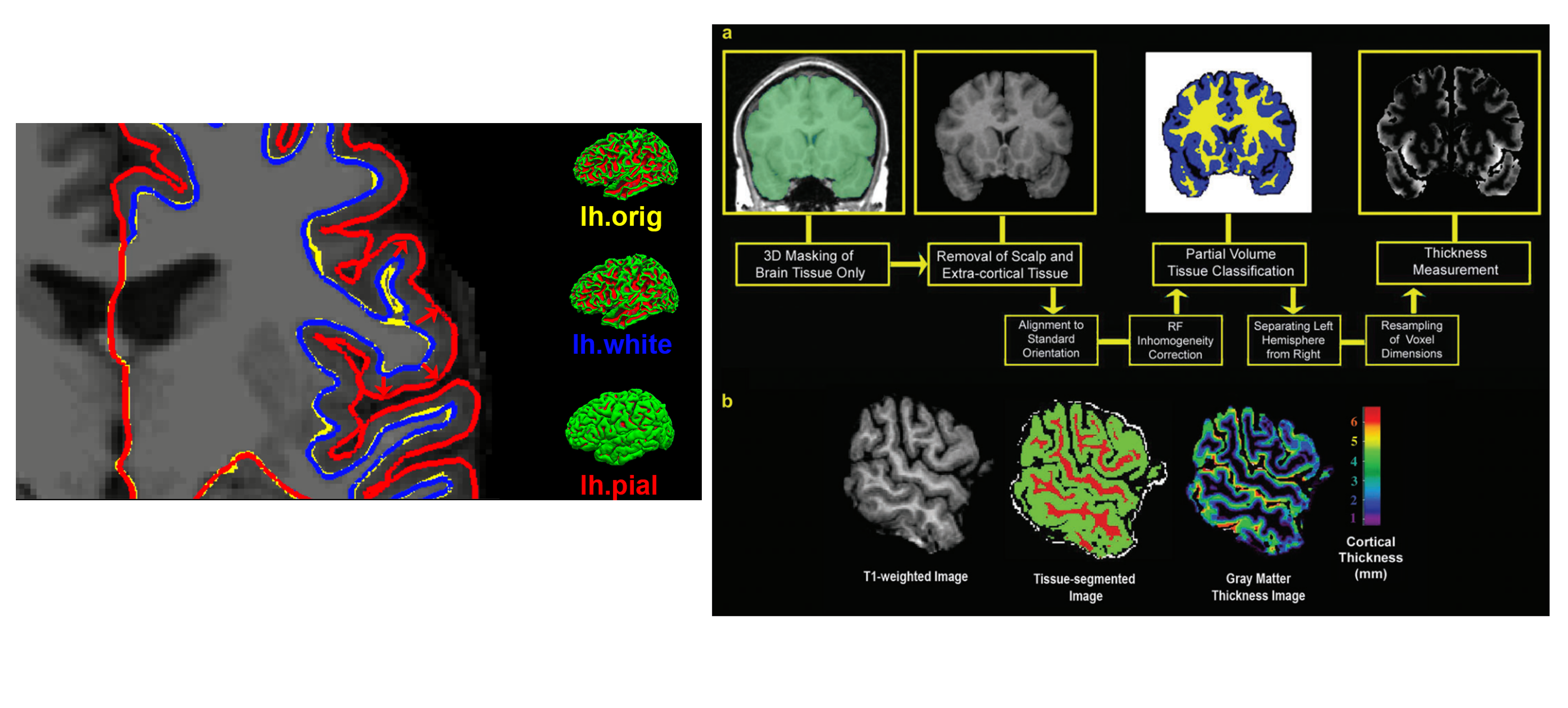
Cortical Thickness
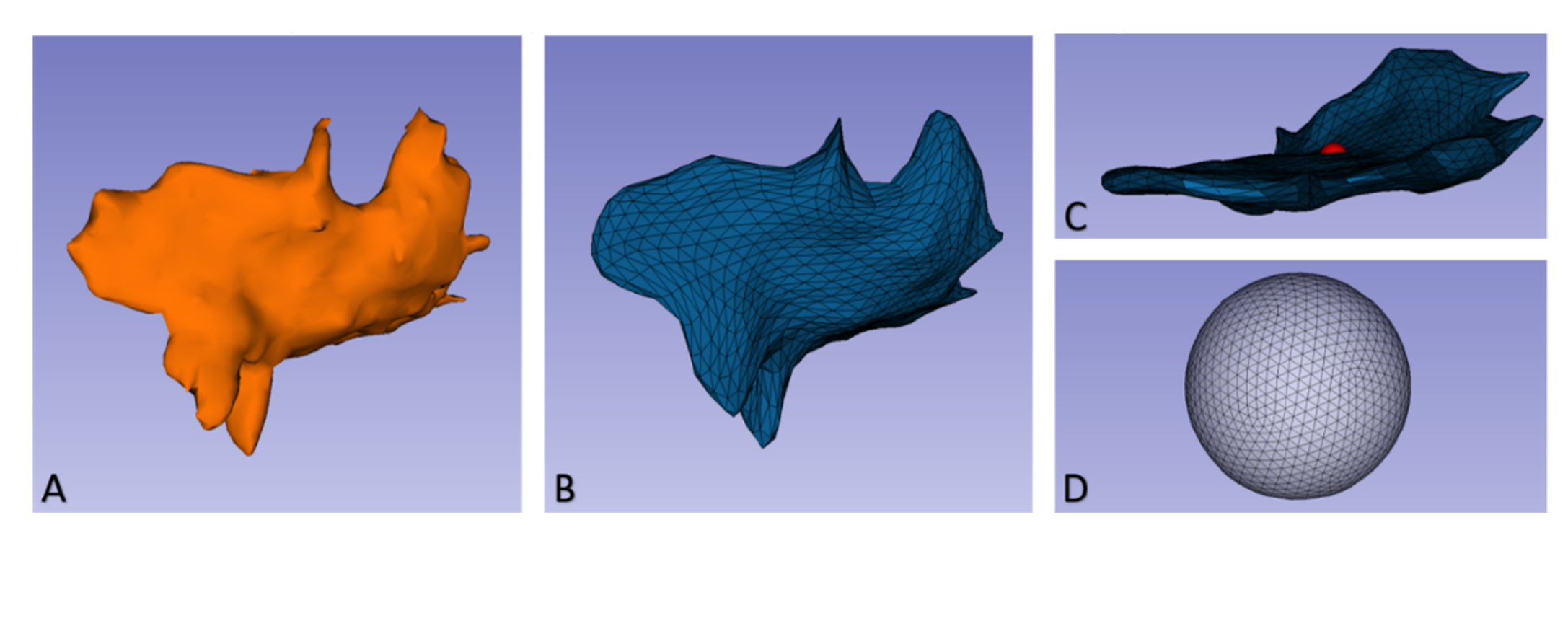
Shape (Morphology)
Structural Connectivity